Alternative Peptide Synthesis Technologies
Peptides and proteins are essential for life and are the foundation for many research fields. Scientists are often interested in protein functions, e.g. to improve the activity of proteins or to understand their mode of action. Protein structure, primarily determined by amino acid sequence, is key to its function and role. Traditional protein synthesis uses biological methods limited to twenty natural amino acids, restricting structure manipulation. Chemical synthesis, on the other hand, allows introducing non-natural amino acids, broadening potential structures and functions. However, Solid Phase Peptide Synthesis (SPPS), the conventional chemical method, is limited to shorter peptide chains (up to 60 amino acids) due to organic solvents and protective group requirements. To solve this problem we will develop an alternative peptide synthesis technology that is inspired by biosynthesis pathways found in nature.
Contact: Jordy Saya
Timeline: 01/06/2024 – 31/05/2028
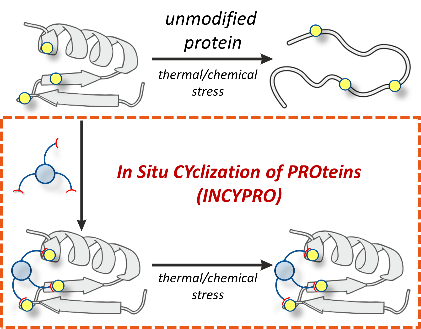
Alternative in-situ cyclization of proteins (INCYPRO)
Proteins are nature-derived molecules that have found wide applications in biotechnology, pharmaceuticals and biocatalysis. A major limitation for the use of proteins in such applications is their lack of stability. This is due to the disruption of the tertiary structure of a protein, which is responsible for the function of a protein. Earlier studies have shown that three cysteine residues, which are strategically incorporated in the sequence, can be crosslinked with a tris-electrophile. This so-called in situ cyclization of proteins (INCYPRO) results in the rigidification of the protein structure and ultimately higher stability of the protein. For some proteins, it would be beneficial to use another amino acid residue for modification as cysteine residues can be important for the function of a protein, e.g. as disulfide bridges or as active sites residues.
NWO KIEM GoChem
Contact: Jordy Saya
Timeline: 01/04/2023 – 31/03/2024

Sustainable Production Routes for Complex Medical Hydrogels using Biomass
The scientific targets for the project are to establish sustainable conversion pathways to tailor specific functional polysaccharides (BDC), explore new post-modification techniques to introduce photo-sensitive side groups into polysaccharides, and to demonstrate biomedical applicability of these functional materials for the fabrication of complex hydrogel (Polymers that do not dissolve in water) constructs by photocrosslinking assisted 3D lithography (AMIBM).
This will initially focus on the isolation of alginates, dextrans and pectins from biomass and subsequent modifications to aryl or acyl azides or bis diazirines.
We anticipate applications from this work as biocompatible materials for lithography-based 3D bioprinting of hydrogel scaffolds in tissue engineering and regenerative medicine.
Contact: Romano Orru
Timeline: 01/01/2023 – 31/12/2023

BM2C6 - Developing a new value chain from biomass to C6 monomers
By means of the new BM2C6 project, we aim to create a unprecedented value chain starting from biomass up to C6 monomers. All process steps will be demonstrated on pilot scale. This project is expected to show both ecologic and economic benefits for the entire value chain. This will give a wide range of opportunities to industrial partners to step in and differentiate themselves in sustainable activities.
InSciTe
Contact : Romano Orru
Timeline: 01/10/2020 – 31/12/2021
https://www.maastrichtuniversity.nl/research/bm2c6
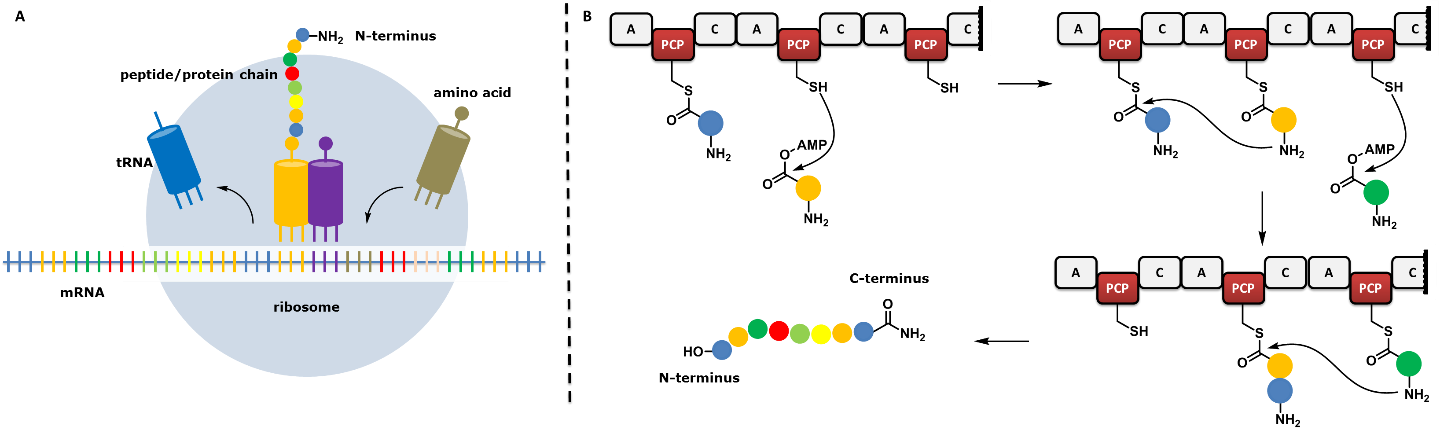
Learning from nature to synthesize peptides and proteins
In this project, we propose an alternative peptide and protein synthesis strategy, inspired by nature, which develops the field in a more sustainable way. Peptides and proteins are large complex molecules made from just a handful of building blocks called amino acids. These essential molecules for life form the basis for many areas of research and in order to study them properly it is important that scientists have access to large quantities. The synthetic approach of nature makes it seem so simple, but the synthetic alternative developed by chemists is far from the same level of elegance and efficiency.
NOW – ENW – XS
Contact: Jordy Saya
Timeline: 01/12/2020 – 31/05/2021
https://www.maastrichtuniversity.nl/research/learning-nature-synthesize-peptides-and-proteins
Circular Cultivation and Chemistry
The agricultural sector is looking for new ways to generate income and improve value chains that contribute to a circular economy, sustainable production and consumption, and healthy food. In this program, we try to realize this focusing on mission-driven innovations. At the same time, the petrochemical industry produces highly developed materials that are under societal and ecological pressure. As we all want to maintain our current standards of living, our society faces the limits of what our ecosystem (including climate issues) can manage in terms of petrochemical production of essential small molecular building blocks necessary for fine chemicals, medicines or high-performance materials.
Interreg Vlaanderen-Nederland
Contact: Romano Orru
Timeline: 01/02/2023 – 31/01/2026
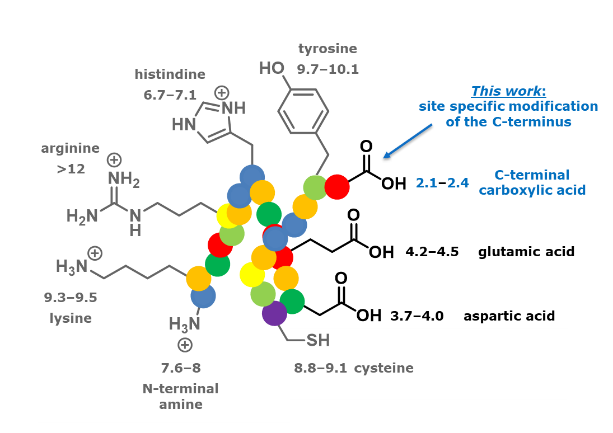
Improving Chemo-Enzymatic Synthesis of Proteins
Chemo-enzymatic peptide synthesis is unique in enabling the fast and sustainable synthesis of cyclic peptides, complex peptides and functionalized mini-proteins. The starting materials are routinely obtained by solid-phase peptide synthesis. One of the starting materials requires an oxo-ester functionality for recognition by the enzymes active site. The SPPS-based synthesis of the oxo-ester functionality still suffers from significant byproduct formation and low overall synthesis yields. The solution to this is introduction of the oxo-ester after obtaining the peptide fragment. In this research we will develop new synthetic methodologies to accomplish this.
NWO KIEM GoChem
Contact: Jordy Saya
Timeline: 01/10/2022 – 30/09/2023
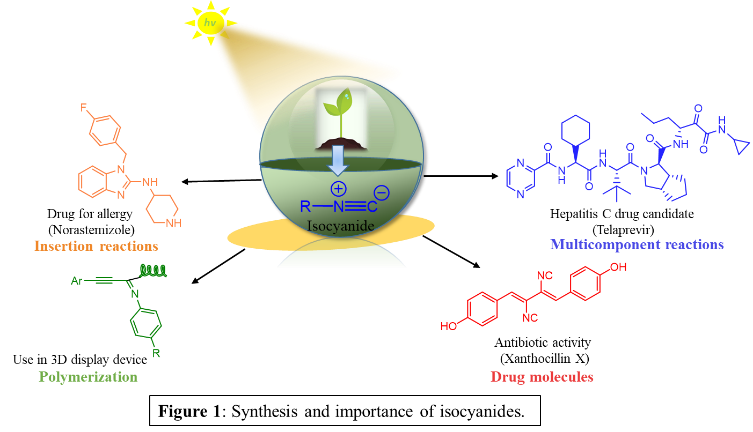
A New Light on Isocyanide Synthesis
The aim of this project is to accomplish an economically viable and ecofriendly large scale synthesis of this fundamental and highly versatile building block that has already found widespread appreciation in academia, but suffers from underutilization in the pharma-, fine-chemicals and materials industry. The most common route to access isocyanides is the dehydration of formamides using various dehydrating agents, like the combination of triethylamine and POCl3/Burgess’s reagent/cyanuric chloride/triflic anhydride/p-tosyl chloride. Even though a wide number of synthetic procedures for isocyanide preparation have been devised over the years, these methodologies still hold a number of challenges outlined below.here.
Marie Skłodowska-Curie fellowship
Contact: Prabhat Ranjan
Timeline: 01/01/2022 – 31/12/2023

