|
Vincent Reinartz |
|
| PhD Student | |
| Room: |
200.3.054 |
| Email: | |
| LinkedIn: | |
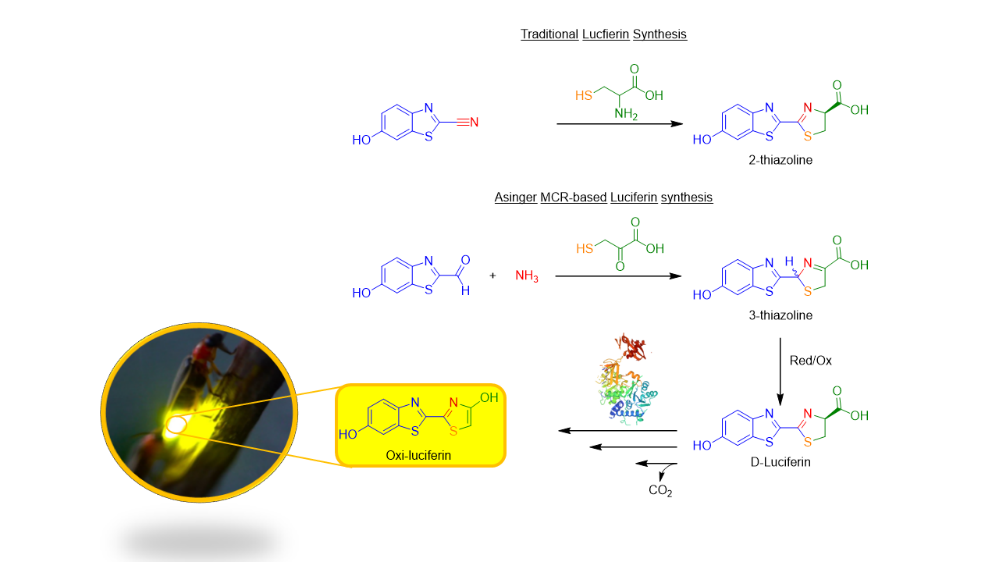
Applying the Asinger MCR in the modular synthesis of luciferin analogues
Luciferin found in nature has been applied in various well-established methods for bio-imaging due to their chemiluminescent properties. In recent years, significant developments have been made to synthesize various luciferin analogues in order to fine-tune the luminescent output for certain bio-luminescent imaging techniques. Most of the current synthesis routes for luciferin analogues follow similar multi-step convergent syntheses with an overall poor atom economy. Multicomponent reactions (MCRs) have been recognized in the synthetic community as a useful tool to tackle synthetic problems and access molecules of interest. Their special characteristics such as their convergence, sustainability, the very large chemical and scaffold space that they cover, the generation of high complexity and diversity offer great opportunities. Friedrich Asinger was a pioneering chemist that developed a multicomponent reaction involving: elemental Sulphur, gaseous ammonia and α-ketones/aldehydes to provide access to novel Δ3-thiazolines. However, has still been underexplored for its potential. This research aims to apply the aforementioned reaction in the synthesis of luciferin analogues and could result in a: modular and versatile reaction methodology for the synthesis of fine-tuned luciferin analogues. Being applied in novel bio-and luciferase-labelled cell imaging and material applications.
- Noordzij, G.J.; Roy, M.; Bos, N.; Reinartz, V.; Wilsens, C.H.R.M. Improving the Hydrolysis Rate of the Renewable Poly(hexamethylene sebacate) Through Copolymerization of a Bis(pyrrolidone)-Based Dicarboxylic Acid. Polymers 2019, 11, 1654.